The London-based medtech company recently completed its submission based on meeting OSPREY’s primary safety and efficacy endpoints. It submitted six-month data and provided 12-month interim results with plans to share the full 12-month dataset during the review.
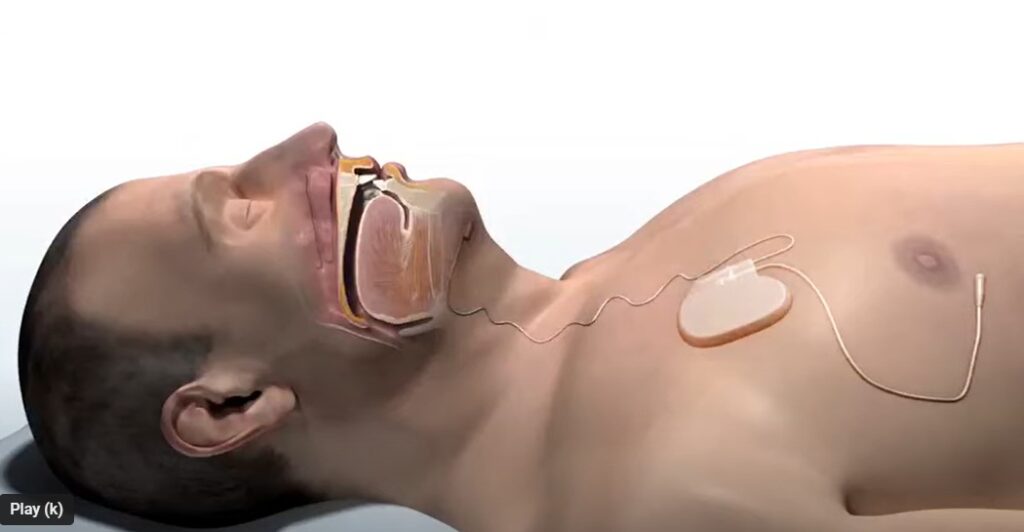
aura6000 is an investigational implantable proximal hypoglossal neurostimulator. LivaNova hopes to bring it to market to treat adult patients with moderate to severe obstructive sleep apnea (OSA). If successful, the implant could rival Inspire Medical’s neurostimulator as an alternative to traditional CPAP sleep respiratory systems.
Year-long data shared today by LivaNova highlighted a 65% responder rate in the treatment arm, with responders defined as those who realized at least a 50% improvement from the baseline apnea-hypopnea index (AHI) and an AHI value below 20. The study features a differentiated neurostimulation modality called proximal hypoglossal nerve stimulation (p-HGNS). It utilizes six electrodes placed on the proximal trunk of the hypoglossal nerve for broad access to the muscles controlling the airway.