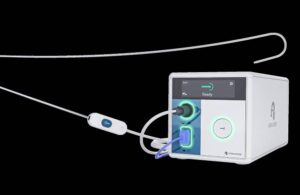
Hotwire is a novel radiofrequency guidewire left-heart access device. It enables zero exchange left-heart access while acting as a rail for catheter-based therapy systems. Steven Mickelsen, a pioneer in the pulsed field ablation space, invented the Hotwire system with Atraverse co-founder Eric Sauter.
The novel guidewire device initially received the FDA’s green light in May 2024. Now, FDA clearance includes the system’s Hotwire RF (radiofrequency) generator, integrated with the guidewire. The generator mitigates the risk of uncontrolled energy delivery after accessing the left atrium. It can also activate energy within the sterile field to give clinicians more precise control.