Researchers identify new method to protect against sepsis
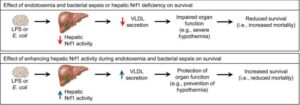
A team of University of Saskatchewan (USask) researchers have identified a pathway to help the human body defend itself against sepsis—a life-threatening condition caused by the body’s inappropriate response to an infection.