
FDA clears Spectrum Dynamics’ Veritas.AI platform
Veritas.AI is designed to advance the functionality of Spectrum Dynamics’ VERITON-CT scanner for nuclear imaging.

Veritas.AI is designed to advance the functionality of Spectrum Dynamics’ VERITON-CT scanner for nuclear imaging.
Rystiggo is administered weekly, 3ml to 6ml over 15 to 30 minutes for six weeks.
MENLO PARK, Calif., Jan. 29, 2026 /PRNewswire/ — GRAIL, Inc. (Nasdaq: GRAL), a healthcare company whose mission is to detect cancer early when it can be cured, today announced the submission of the final module of the Premarket Approval (PMA) application to the U.S. Food and Drug Administration (FDA) for its Galleri® multi-cancer early detection (MCED) test. The FDA designated the test as a Breakthrough Device in 2018.
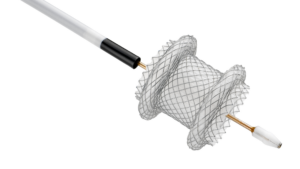
ORANGEBURG, S.C., Jan. 29, 2026 /PRNewswire/ — Zeus, a global leader in advanced polymer solutions, today announced the launch of PFX™, a breakthrough platform designed to advance catheter innovation with a focus on performance, design flexibility, and sustainability.

SEOUL, South Korea, Jan. 29, 2026 /PRNewswire/ — NEXTBIOMEDICAL CO., LTD. (KOSDAQ: 389650), an innovative medical device company based in South Korea, announced today that Nexsphere-F™, the company’s novel fast resorbable microsphere for musculoskeletal pain embolization has received approval from Health Canada.

PITTSBURGH, Jan. 29, 2026 /PRNewswire/ — MolecuLight today announced that its MolecuLightDX® wound measurement has been qualified by the U.S. Food and Drug Administration (FDA) as a Medical Device Development Tool (MDDT). The FDA’s MDDT program qualifies select, scientifically validated tools for use in medical device development and evaluation, enabling sponsors to generate reliable, FDA accepted data in clinical investigations.

SARASOTA, Fla., Jan. 29, 2026 /PRNewswire/ — Spectrum Dynamics Medical, a global leader in digital nuclear medicine imaging solutions, today announced that it has received FDA 510(k) clearance for Veritas.AI™ Noise Reduction, its advanced artificial intelligence platform designed to significantly enhance image quality, diagnostic confidence, and operational efficiency on the VERITON-CT® digital SPECT/CT system.

YOUNGSTOWN, Ohio, Jan. 29, 2026 /PRNewswire/ — Nivalon Medical Technologies Inc. has successfully produced the world’s first fully patient-specific, motion-preserving spinal implant built entirely without metal, using AI-driven design and advanced ceramic 3D printing.
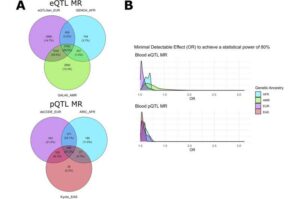
Numerous genetic studies have identified many risk variants for type 2 diabetes (T2D)—but which genes and proteins are actually involved in the disease mechanisms?

Ultrasonography is a noninvasive imaging technique used for real-time imaging. This versatile technique is used as a reliable diagnostic tool in various modalities.