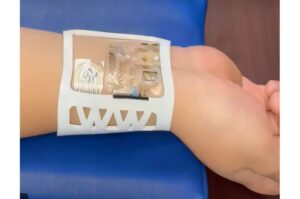
Bioelectronic wristband offers continuous, objective, real-time stress monitoring
Researchers at the University of California, Irvine have developed a multimodal, bioelectronic wrist-worn device for objective, continuous, real-time monitoring of stress.