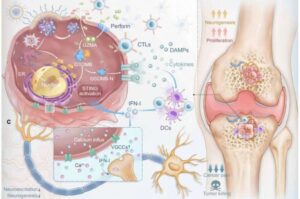
New nanotherapy eases bone metastasis pain by disrupting tumor-nerve crosstalk
A new nano-sized drug carrier that finds bone tumors and releases treatment exactly where it’s needed is here to improve the precision and comfort of cancer therapy.

A new nano-sized drug carrier that finds bone tumors and releases treatment exactly where it’s needed is here to improve the precision and comfort of cancer therapy.

Glaukos (NYSE:GKOS) announced today that the FDA approved a new drug application (NDA) labeling supplement for its iDose TR system.

Large study of patients in U.S., Colombia, Nigeria and India finds symptom burden highest in high-income countries
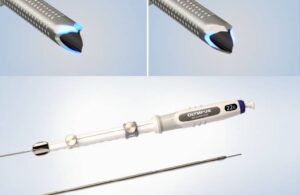
Olympus today announced the U.S. launch of its most advanced single-use fine needle biopsy device.
eMurmur has received FDA 510(k) clearance for its next-generation heart murmur detection software, eMurmur Heart AI (2.2).
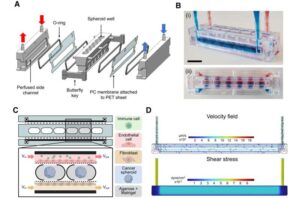
Cancer research laboratory tests can now be done using micro-physiological systems mimicking human physiology.
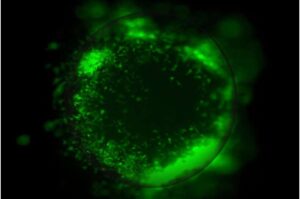
UCLA scientists have developed advanced miniature 3D tumor organoid models that make it possible to study glioblastoma tumors in a setting that closely mirrors the human brain, shedding light on how the aggressive cancer interacts with surrounding brain cells and the immune system to become more invasive and resistant to therapy.
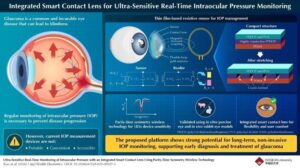
Glaucoma is a leading cause of blindness among people who are unable to monitor and manage their intraocular pressure (IOP) daily. The current tools for IOP measurement are not portable, convenient, easily accessible, or capable of continuous (24/7) monitoring.

The conventional approach to studying and treating these episodes is to focus on the heart as an isolated organ. University of California San Diego research, led by the School of Biological Sciences, is upending the way heart attacks are viewed under a transformative new understanding of how cardiac events are interconnected with other systems.

Gene-editing tools like CRISPR have unlocked new treatments for previously uncurable diseases. Now, researchers at the University of British Columbia are extending those possibilities to the skin for the first time.