
Stimvia plans to enter neuromodulation market in the USA after MDR certification
The Czech company says this is the first non-invasive closed-loop neuromodulation system to treat overactive bladder (OAB) without surgery or drugs.

The Czech company says this is the first non-invasive closed-loop neuromodulation system to treat overactive bladder (OAB) without surgery or drugs.

Dexcom (Nasdaq: DXCM)+
today announced a significant product launch as another continuous glucose monitor (CGM) is hitting the market.
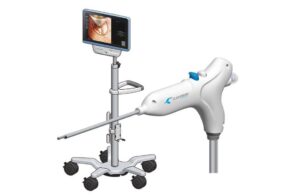
Clearmind Biomedical announced today that it received FDA 510(k) clearance for its Neuroblade neuroendoscopy system.
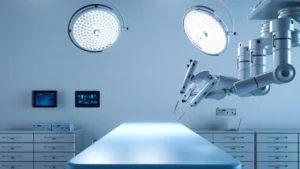
SUNRISE, Fla., Aug. 26, 2024 /PRNewswire/ — Obvius Robotics, a medical device company developing an innovative technology platform for democratizing vascular access, today announced the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for its CERTA Access System for central venous catheterization (CVC).

The FDA clearance marks the first automated insulin delivery system for people with Type 2 diabetes.

WESTBOROUGH, Mass., Aug. 26, 2024 /PRNewswire/ — Olympus Corporation, a global medical technology company committed to making people’s lives healthier, safer, and more fulfilling, announced today the launch of two new jaw designs in the POWERSEAL™ Sealer/Divider family of advanced bipolar surgical energy products: the POWERSEAL Straight Jaw, Double-action (SJDA) and the POWERSEAL Curved Jaw, Single-action (CJSA). The first POWERSEAL device, launched in 2021, is the POWERSEAL Curved Jaw, Double-action (CJDA), which has established a strong foothold for Olympus in the advanced bipolar surgical energy market.

RENO, Nev. and ROCKVILLE, Md., Aug. 23, 2024 /PRNewswire/ — Melzi Surgical, a medical device company focused on providing innovative technology to locate lost surgical sharps and Spartan Medical Inc., a veteran-owned medical solutions company, have launched a campaign to raise awareness around the impacts of Retained Surgical Items (RSIs) and near-misses to provide a unique offering to reduce RSIs and potential consequences.
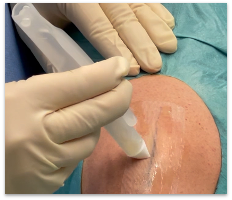
AKRON, Ohio, Aug. 23, 2024 /PRNewswire/ — Resivant Medical announced today it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its first two products, Cutiva™ Topical Skin Adhesive, and Cutiva PLUS™ Skin Closure System, which combines an adhesive mesh patch with high-viscosity Cutiva™ liquid adhesive. This achievement represents a significant advancement in medical tissue adhesive and wound closure technology and marks the first major adhesive advancement in over 25 years.
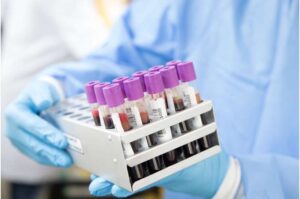
A paper published in Alzheimer’s Research & Therapy outlines a study undertaken by the Institute of Memory & Cognition at Tallaght University Hospital (TUH) which could change the way Alzheimer’s disease is detected.
SurGenTec announced today that it received FDA 510(k) clearance for its proprietary B-MAN bone marrow aspirate kit.