FDA clears Oticon Medical’s hearing loss implant
Oticon Medical announced today that the FDA granted clearance for its Sentio transcutaneous bone conduction hearing system.
Oticon Medical announced today that the FDA granted clearance for its Sentio transcutaneous bone conduction hearing system.

RA’ANANA, Israel, July 11, 2024 /PRNewswire/ — Inspira™ Technologies OXY B.H.N. Ltd. (Nasdaq: IINN) (Nasdaq: IINNW) (the “Company” or “Inspira”), a breakthrough medical technology company, is excited to announce the receipt of the Israeli Ministry of Health’s medical devices and accessories (“AMAR”) approval for the INSPIRA™ ART100, an Extra-Corporeal Membrane Oxygenation and Cardiopulmonary Bypass system. This is a pivotal milestone in Inspira’s strategy to conduct business development activities to bring its innovative products and technologies to the market.

Two cellular defects appear to drive the autoimmune disease that affects more than 1.5 million Americans

REMSleep Holdings Inc has received FDA 510(K) clearance for its new DeltaWave nasal pillow CPAP interface mask. The DeltaWave is designed to enhance comfort and maintain natural breathing patterns, aiming to address common issues that lead to therapy discontinuation

Bryan Hsu is tackling an area of research that has long been neglected—menstrual products.

FORT LAUDERDALE, Fla., July 10, 2024 /PRNewswire/ — Wounds can create malodorous smells in various settings and for a variety of reasons. For example, wound malodor can develop due to several healthcare conditions, including the treatment of tumoral wounds, pressure ulcers and sores, diabetic foot, burns, and post-surgery wounds

ImmersiveTouch announced today that it received FDA 510(k) clearance for its ImmersiveAR augmented reality technology platform.
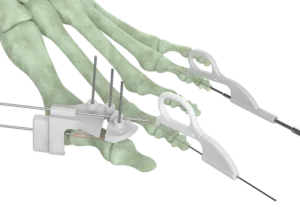
CAMP HILL, Pa.–(BUSINESS WIRE)–Forma Medical Inc., a leading innovator in medical device technologies, is thrilled to announce the FDA 510(k) clearance for its groundbreaking OptimalMTP® Plating System. This regulatory milestone marks a significant leap forward in MIS (Minimally Invasive Surgery), ushering in a new era of precision and efficiency dedicated to foot and ankle surgery, one of the fastest growing segments in the medical device space.

To bring light’s benefits to these harder-to-access cancers, engineers and scientists at the University of Notre Dame have devised a wireless LED device that can be implanted. This device, when combined with a light-sensitive dye, not only destroys cancer cells, but also mobilizes the immune system’s cancer-targeting response.
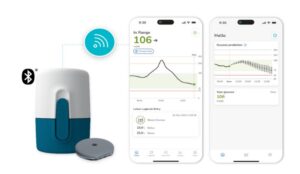
Roche has announced that it has received the CE Mark for its Accu-Chek SmartGuide continuous glucose monitoring (CGM) solution. This significant milestone paves the way for the solution to be made available to people living with type 1 and type 2 diabetes over the age of 18 on flexible insulin therapy.