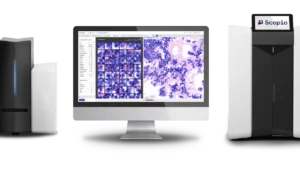
Scopio Labs wins de novo nod for bone marrow analysis software
Scopio can now add the application to its imaging platforms that allow users to view blood samples digitally rather than under a microscope.

Scopio can now add the application to its imaging platforms that allow users to view blood samples digitally rather than under a microscope.

Lumicell today announced it received FDA approval for its LumiSystem direct visualization system for breast cancer removal.

The company’s 3rd FDA clearance accelerates the shift to AI-powered digital hematopathology, enhancing diagnostic speed and patient care

IRVING, Texas, April 16, 2024 /PRNewswire/ — Xstim, Inc., a pioneering developer and manufacturer of cutting-edge bone growth stimulation systems, is thrilled to announce its recent Premarket Application (PMA) approval from the U.S. Food and Drug Administration (FDA) for Xstim™ Spine Fusion Stimulator
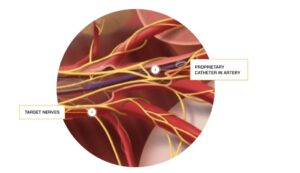
Patent portfolio with over 100 patents, issued and pending, protects technology targeting a $100 billion market opportunity

The 3D printed PEEK implants using the EXT 220 MED were successfully demonstrated in nearly 40 cranioplasties across Europe in recent months says the company.

FastWave Medical announced today that it received a fourth utility patent to strengthen the IP foundation of its IVL platform.
Simpson Interventions announced this week that it received FDA breakthrough designation for its Acolyte image-guided crossing and re-entry catheter system.

Getinge announced today that it received EU MDR certification for its Advanta V12 covered stent system for patients with aortoiliac occlusive disease (AIOD)

Gynecological health startup Daye has announced that its diagnostic tampon, which provides a non-invasive method of screening your vaginal health at home, is now available to buy.