
MIT engineers create a chip-based tractor beam for biological particles
The tiny device uses a tightly focused beam of light to capture and manipulate cells.

The tiny device uses a tightly focused beam of light to capture and manipulate cells.

Eyenovia (Nasdaq:EYEN) announced that it began manufacturing batches of its Mydcombi product for its next-generation delivery platform.
SetPoint Medical today announced FDA Investigational Device Exemption approval to study its proprietary neuroimmune modulation platform for people with relapsing-remitting multiple sclerosis.
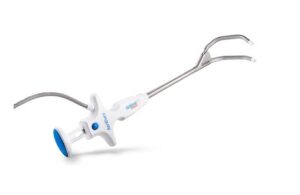
AtriCure (Nasdaq: ATRC)+
announced that it received CE mark for the EnCompass clamp for cardiac tissue ablation procedures.
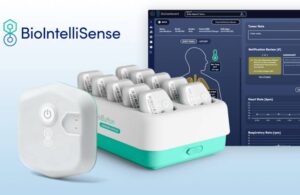
BioIntelliSense announced today that it received FDA clearance for its rechargeable BioButton multi-patient wearable and BioDashboard system.
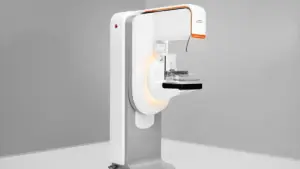
The 3D capabilities are part of Siemens’ first completely redesigned mammography platform in over a decade.

Qnovia announced that the FDA cleared its investigational new drug (IND) application for the RespiRx smoking cessation inhaler (QN-01).
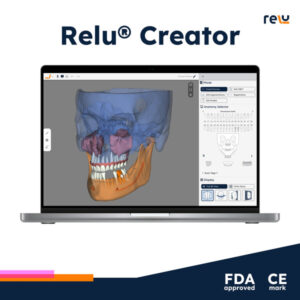
LEUVEN, Belgium, Oct. 1, 2024 /PRNewswire/ — Relu, a pioneer in artificial intelligence (AI) assisted segmentation for dental labs and software companies, proudly announces the dual achievement of 510(k) clearance by the U.S. Food and Drug Administration (FDA) and CE Mark approval by an EU Notified Body. These regulatory milestones authorize the commercial distribution of the Relu® Creator, the cutting-edge dental tool that enables users to create 3D anatomical models from patients in just minutes.

Surmodics (Nasdaq:SRDX) announced today that it received FDA 510(k) clearance for its Pounce XL thrombectomy system.

Called Flyrcado, the radiotracer could help improve cardiac imaging accuracy in patients with a high body mass index and women, the company said.