
Axena Health receives two additional positive insurance coverage decisions for Leva
Highmark BCBS and BCBS of North Dakota cover the Leva Pelvic Health System for women seeking effective treatment for urinary incontinence

Highmark BCBS and BCBS of North Dakota cover the Leva Pelvic Health System for women seeking effective treatment for urinary incontinence

The Food and Drug Administration (FDA) has approved the Investigational Device Exemption (IDE) of the Lumerah™ technology for the early detection of fetal distress during labor and delivery.

Onkos Surgical this week announced it received FDA de novo approval for its antibacterial coated implants.
Vivos Therapeutics specializes in the development and commercialization of highly effective proprietary treatments for sleep related breathing disorders (including all severities of obstructive sleep apnea (OSA) in adults).
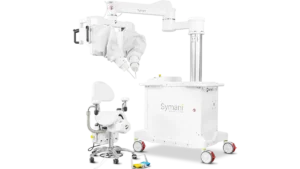
Medical Microinstruments said the system could increase the number of physicians who can perform complicated microsurgical procedures.

Neurovalens announced today that it received FDA clearance for its Modius Stress device for treating anxiety and raised $2.65 million.
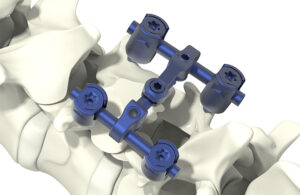
Orthobond has secured FDA de novo approval for its Ostaguard antibacterial technology that could one day be used for a wide range of medical devices — and beyond medtech.
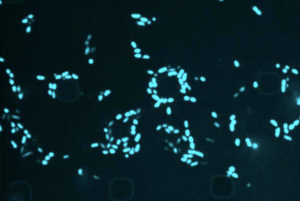
Most antibiotics target metabolically active bacteria, but with artificial intelligence, researchers can efficiently screen compounds that are lethal to dormant microbes.

Siemens Healthineers announced today that it received FDA 510(k) clearance for its MammoMat B.brilliant mammography platform.

New Indication for Treatment of Pulmonary Embolism Enhances Device Utility in Critical Medical Scenarios