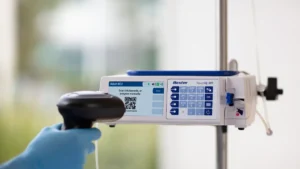
Baxter receives FDA clearance for delayed Novum IQ infusion pump
The clearance ends a three-year back-and-forth with the FDA to get the product to market.

The clearance ends a three-year back-and-forth with the FDA to get the product to market.
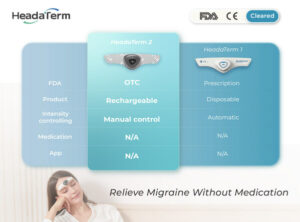
Using neuromodulation technology, HeadaTerm 2 releases targeted electrical impulses to increase pain tolerance of its users. It is FDA-cleared for the preventative treatment of migraine headaches.

NanoDrop is the First Over-the-Counter Blood-Lancing Device Labeled for the Upper Arm to be Cleared in the United States, Changing the Landscape of the At-Home Consumer Testing Market and Decentralized Clinical Trials
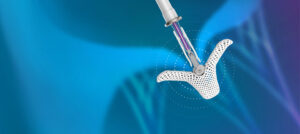
TriClip offers a remarkably safe, minimally invasive treatment option for patients in need of tricuspid valve repair but who are unable to withstand surgery
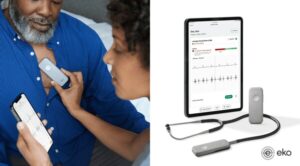
Developed with Mayo Clinic, Eko Health’s Low Ejection Fraction (Low EF) AI gives healthcare professionals a powerful tool to more accurately assess possible heart failure in at-risk patients during a standard physical exam

Ross Bjella, Kelyniam’s CEO, said, “This approval will further boost Kelyniam’s sales momentum which started in Q4 last year

Researchers from the University of Birmingham have received funding from Cancer Research UK and the Engineering and Physical Sciences Research Council to create a new ‘lollipop’ diagnostic for mouth cancer using a novel smart hydrogel.

The FDA’s Investigational Device Exemption (IDE) clears the way for a pivotal study of Fractyl’s Revita system designed to maintain weight loss after patients have stopped taking GLP-1 drugs
Baird Medical Devices, Inc. today announced it has been granted a new Class III certificate by the National Medical Products Administration (NMPA) in China

The company is pitching the test as a way to improve the safety and availability of blood.