
FDA clears 5008X hemodialysis system from Fresenius Medical Care
Fresenius Medical Care announced today that it received FDA clearance for its 5008X hemodialysis system.

Fresenius Medical Care announced today that it received FDA clearance for its 5008X hemodialysis system.

FDA backing for the Wavewriter SCS systems allows the company to challenge Abbott and Nevro.

New technique made human T cells 100 times more potent at killing cancer cells

BETHLEHEM, Pa., Feb. 6, 2024 /PRNewswire/ — Tyber Medical LLC, a leading orthopedic device manufacturer providing private label orthopedic implants for the trauma, extremity, and spine markets, received clearance for the anatomical plating system in Canada. The comprehensive portfolio previously received FDA 510(k) in the US and has now been cleared through Health Canada.

ROSWELL, Ga., Feb. 6, 2024 /PRNewswire/ — StimLabs® announces FDA 510(k) clearance of Corplex P, the pioneering human umbilical cord-derived medical device. This first-of-its-kind clearance marks a significant milestone for the wound care industry and highlights StimLabs’ position at its forefront.

Boston Scientific (NYSE: BSX)+
announced today that the FDA approved an expanded indication for its WaveWriter spinal cord stimulation (SCS) systems.
Philips (NYSE: PHG)+
announced today that it received FDA 510(k) clearance for its latest IntelliVue patient monitor software.
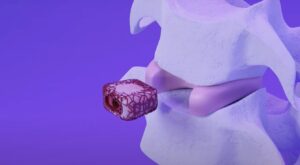
CARLSBAD, Calif., Feb. 05, 2024 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG), a designer and manufacturer of innovative medical devices that improve spinal surgery outcomes, today announced the issuance of its second United States Patent No: 11,850,162 entitled “Body Density Scan Result-Matched Orthopedic Implants and Methods of Use” for The World’s First DEXA Technology™ Patient-Matched Implant Technology.
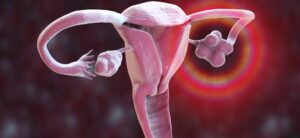
Roche Diagnostics has announced CE mark approval for a claim extension to the Elecsys Anti-Müllerian Hormone (AMH) Plus immunoassay. The claim extension means that the blood test, already available on the NHS, can now be used by physicians to help diagnose women suspected of having polycystic ovary syndrome (PCOS) as an alternative to a transvaginal ultrasound.

Hologic (Nasdaq: HOLX)+
announced that the FDA granted clearance for its Genius digital diagnostics system with the Genius cervical AI algorithm.