
iRhythm wins CE mark for next-gen Zio ECG system, Zeus software
iRhythm Technologies (Nasdaq: IRTC)+
announced today that it received CE mark for its Zio system and its supporting Zeus AI algorithm.

iRhythm Technologies (Nasdaq: IRTC)+
announced today that it received CE mark for its Zio system and its supporting Zeus AI algorithm.

EndoSound announced today that it received FDA 510(k) clearance for its EndoSound Vision System (EVS).
FDA clearance supported by highly compelling clinical study results demonstrating 93% of subjects treated arms were “improved” or “very much improved” in appearance, following treatment utilizing SUPERB™
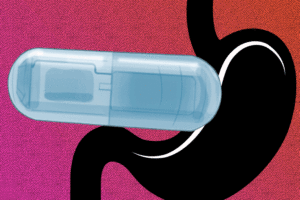
Swallowing the device before a meal could create a sense of fullness, tricking the brain into thinking it’s time to stop eating.
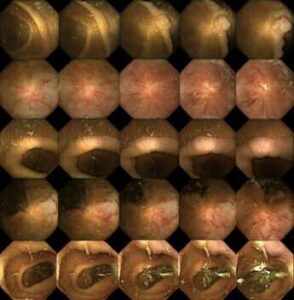
Thanks to new technology, a tiny capsule camera can examine your intestines while you get on with your work or walk your dog. Compared with the alternatives, patients experience virtually no discomfort at all.

Johnson & Johnson MedTech unit Acclarent announced that it won a new FDA clearance for its Aera Eustachian tube balloon dilation system.
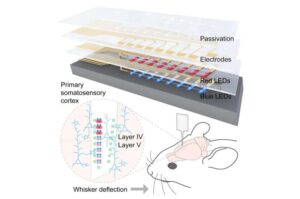
A team of researchers at the University of Massachusetts Amherst has developed the first dual-color optoelectronic neural probe.
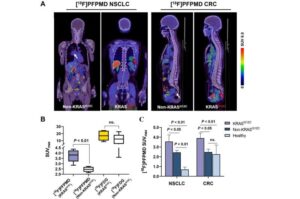
A novel PET imaging tracer has been proven to safely and effectively detect a common cancer gene mutation that is an important molecular marker for tumor-targeted therapy. By identifying this mutation early, physicians can tailor treatment plans for patients to achieve the best results. This research was published in the December issue of the Journal of Nuclear Medicine.

Because it was not possible to remove a hard-to-reach bulge in the central lymphatic system, a team of doctors from the Department of Plastic Surgery and Hand Surgery at the University Hospital Zurich (USZ) created a new drain to relieve the patient’s agonizing lymph congestion. For the first time, a microsurgical operating system was used for such a procedure.
ALWAY, Ireland–(BUSINESS WIRE)– Perfuze, a private medical device company dedicated to developing pioneering technology to treat acute ischemic stroke, proudly announces FDA clearance for the Millipede 070 Aspiration Catheter and the 2nd generation of the Millipede 088 Access Catheter.