
Senseonics receives FDA clearance for one-year CGM
Commercial partner Ascensia is in discussions with insulin pump manufacturers to create an automated insulin delivery system.

Commercial partner Ascensia is in discussions with insulin pump manufacturers to create an automated insulin delivery system.

Biotronik announced today that it received FDA approval for its Selectra 3D catheter and Solia S lead for use in left bundle branch area pacing (LBBAP).

The company says that Lightning Flash 2.0 will be the most advanced thrombectomy system on the European market to address venous and pulmonary thrombus.

Johnson & Johnson MedTech‘s Shockwave Medical today announced the full U.S. launch of its Shockwave E8 peripheral IVL catheter.
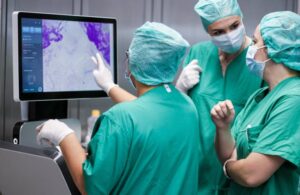
SamanTree Medical announced today that it received FDA 510(k) clearance for its Histolog scanner for tissue imaging.

SILVER SPRING, Md., Sept. 12, 2024 /PRNewswire/ — Today, the U.S. Food and Drug Administration authorized the first over-the-counter (OTC) hearing aid software device, Hearing Aid Feature, intended to be used with compatible versions of the Apple AirPods Pro headphones. Once installed and customized to the user’s hearing needs, the Hearing Aid Feature enables compatible versions of the AirPods Pro to serve as an OTC hearing aid, intended to amplify sounds for individuals 18 years or older with perceived mild to moderate hearing impairment.

AUSTIN, Texas, Sept. 12, 2024 /PRNewswire/ — NeuFit is revolutionizing neurological rehabilitation with its innovative approach that integrates advanced diagnostics and therapeutic technologies. At the center of NeuFit’s success is the NEUBIE (Neuro-Bio-Electric Stimulator), a cutting-edge device that utilizes direct current (DC) electrical stimulation to promote accelerated recovery, enhance performance, and improve overall well-being. This press release highlights NeuFit’s unique approach and its multi-site clinical trial collaboration with Hands-On Diagnostics (HODS) to address neuropathy.

Researchers at the University of Pittsburgh are pioneering a new approach to blood pressure monitoring—using the devices we carry with us every day.

“We are adding a new layer of control between the world of computers and what your eyes see,” says Barmak Heshmat, co-founder of Brelyon and a former MIT postdoc.

iRhythm Technologies (Nasdaq: IRTC)+ announced that it received approval in Japan for its Zio long-term continuous monitoring (LTCM) system.