MedTech News
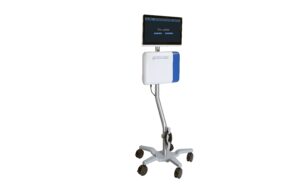
Provisio Medical Announces FDA Clearance of the Provisio SLT IVUS™ System
Groundbreaking technology for the precise, automatic measurement of peripheral blood vessels to optimize treatment strategies
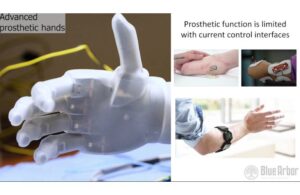
Blue Arbor wins FDA breakthrough nod for neuromuscular interface
Blue Arbor Technologies announced today that it received FDA breakthrough device designation for its Restore neuromuscular interface system.

FDA clears Sail Fusion’s BowTie Sacroiliac Fusion System
Sail Fusion today announced it received FDA 510(k) clearance for its BowTie Sacroiliac Fusion System.
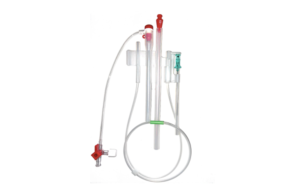
Front Line Medical wins CE mark for COBRA-OS device
Front Line Medical Technologies today announced it received CE mark approval for its COBRA-OS device.

FDA clears computer-assisted thrombectomy system from Penumbra
Penumbra (NYSE:PEN) announced today that it received FDA clearance for and began the launch of its Lightning Flash 2.0 CAVT system.

Innovative Aspiration Thrombectomy System by Expanse ICE Receives FDA Clearance for vessels of the peripheral arterial and venous systems
PLEASANTON, Calif., April 22, 2024 /PRNewswire/ — Expanse ICE announced today the ICE Aspiration System has received 510(k) clearance from the U.S. Food and Drug Administration. This announcement introduces a new and exciting player in the peripheral thrombectomy market.
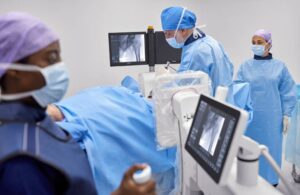
FDA clears new Zenition 30 mobile C-arm from Philips
Philips (NYSE: PHG)+
announced today that the FDA granted 510(k) clearance for its Zenition 30 mobile C-arm.
FDA approves Lumicell’s breast cancer imaging tool
Lumicell developed the technology to help physicians detect residual cancer in the breast cavity after surgery.
