MedTech News

Tenovi’s FDA-Cleared Blood Pressure Monitor Advances Maternal Health and Stroke Prevention
Connected monitoring device brings real-time, remote care support for pregnant patients and individuals at cardiovascular risk.

Phraxis Secures FDA Approval for EndoForce™ Connector to Revolutionize Dialysis Access
Breakthrough endovascular device advances minimally invasive arteriovenous graft creation for patients requiring hemodialysis.
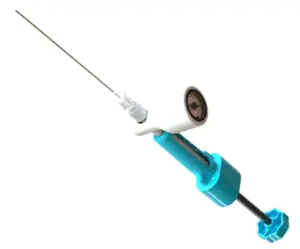
ReGelTec’s HYDRAFIL® System Gains CE Mark Approval for Chronic Low Back Pain Treatment
Breakthrough Injectable Gel Offers Minimally Invasive Relief for Patients with Degenerative Disc Disease Across Europe.

Auxilium Biotechnologies launches pivotal trial for NeuroSpan Bridge implant
Auxilium Biotechnologies today announced enrollment of the first patient in a pivotal trial for its NeuroSpan Bridge implant for nerve regeneration.

Medtronic earns expanded CE mark for Prevail paclitaxel-coated balloon
Medtronic (NYSE: MDT)+
announced today that it received CE mark for several expanded indications for its Prevail balloon catheter

IdentifySensors Advances AI-Powered Diagnostic Tech with FDA Submission
AI-driven system targets real-time, lab-quality disease detection at the point of care
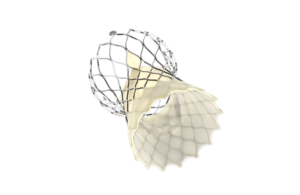
Medtronic earns CE mark for redo TAVI procedure
Medtronic (NYSE: MDT)+
this week announced it received CE Mark approval for an expanded indication of its Evolut Pro+ and Evolut FX TAVI systems, allowing the devices to be used in “redo TAVI” procedures for patients with failing transcatheter aortic valves, regardless of the original manufacturer.

Natus Neuro launches BrainWatch AI-driven, point-of-care EEG
Natus Medical yesterday announced that it launched BrainWatch, a point-of-care EEG solution driven by AI.
