MedTech News
.................... by Andrew Celentano
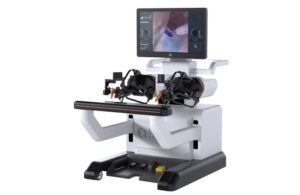
EndoQuest gets FDA green light to initiate final stage of surgical robot trial
EndoQuest Robotics announced today that the FDA approved the initiation of the next and final stage of its PARADIGM trial.

Near-atomic imaging reveals promising target for ‘Brain on Fire’ condition
Scientists have identified a promising target for treatment of a devastating autoimmune disease affecting the brain.
The protein periostin may promote the spread of pancreatic cancer—and pain—through nerves
A new Brazilian study has revealed the key role of the protein periostin and stellate pancreatic cells in allowing pancreatic cancer to infiltrate nerves and spread early, increasing the risk of metastasis.
Blood metabolite signature offers improved prediction of type 2 diabetes risk
Diabetes, a metabolic disease, is on the rise worldwide, and over 90% of cases are type 2 diabetes, where the body does not effectively respond to insulin.
HIF1 protein identified as key trigger in common tendon diseases
Complaints such as pain in the Achilles tendon, tennis elbow, swimmer’s shoulder and jumper’s knee are familiar to many young sportspeople, as well as to older individuals. These conditions are all caused by overloading of tendons and are generally very painful.
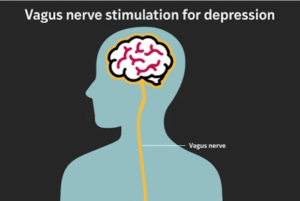
Implant provides lasting relief for treatment-resistant depression, study finds
About 20% of U.S. adults experience major depression in their lifetime. For most people, symptoms improve within a few treatment attempts, but up to one‐third of patients have treatment‐resistant depression, for which standard antidepressant medication or psychotherapy isn’t enough.

Customizable stainless steel neural probes enable safer, less expensive brain sensing
The human brain is complex. Understanding deep brain function usually requires the insertion of probes that frequently result in irreversible tissue damage. Current neural probes are made out of silicon, a brittle material that can shatter during placement.
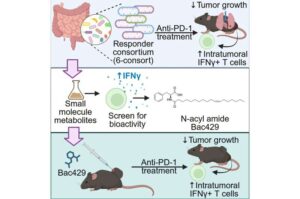
Gut bacteria molecule boosts lung cancer treatment response
UF Health Cancer Institute researchers have discovered a small compound produced naturally by gut bacteria that doubled the response to lung cancer immunotherapy treatment in mice and can now be made into a drug for testing in humans.
