MedTech News
.................... by Andrew Celentano

A simple blood test can predict Crohn’s disease years before symptoms appear
Sinai Health researchers have shown a blood test that can predict Crohn’s disease years before symptoms appear, opening the doors to early diagnosis and potentially prevention.

Scientists report new immune insights and targets into LRRK2 mutations in Parkinson’s disease
Parkinson’s disease (PD) is a debilitating and progressive neurodegenerative disorder caused by the loss of dopamine-producing neurons in the substantia nigra, a brain region essential for motor control. Clinically, it is marked by tremor, rigidity, bradykinesia and postural instability, symptoms that progressively erode independence and quality of life.
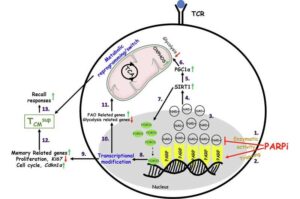
T cells gain superior memory through new reprogramming method, boosting cancer-fighting abilities
Georgetown University’s Lombardi Comprehensive Cancer Center researchers have identified a new way to reprogram T cells, which are infection and tumor-fighting white blood cells, so that they have a superior memory, thereby making them more effective in killing cancer cells.

A new tool could tell us how consciousness works
Consciousness is famously a “hard problem” of science: We don’t precisely know how the physical matter in our brains translates into thoughts, sensations, and feelings. But an emerging research tool called transcranial focused ultrasound may enable researchers to learn more about the phenomenon.
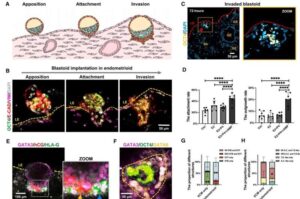
How a miniature womb on a chip can help women struggling to conceive
A team of scientists from China has successfully created a miniature womb on a chip that mimics the complex environment of the human uterus. The research offers a new way to study the exact moment an embryo attaches to a mother’s body.

LEADOPTIK Announces FDA Clearance of the LIA™ for Lung Biopsy Procedures
SAN JOSE, Calif., Jan. 15, 2026 /PRNewswire/ — LEADOPTIK, Inc. today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of its Last Inch Assessment™ (LIA) system, the world’s first system to use silicon photonics imaging technology and software designed to improve the accuracy of lung biopsy procedures.

Neurolief receives FDA PMA Approval for First At-Home Brain Neuromodulation Therapy for Adults Whose Depression Was Not Adequately Improved by Antidepressants
CORAL SPRINGS, Fla., Jan. 12, 2026 /PRNewswire/ — Neurolief Inc., a medical device company focused on neuromodulation therapies for neuropsychiatric conditions, today announced that the U.S. Food and Drug Administration (FDA) has approved Proliv™Rx, the first prescription, physician-directed, at-home brain neuromodulation therapy as an adjunctive treatment for adults with Major Depressive Disorder (MDD) who failed to achieve satisfactory improvement from at least one previous antidepressant medication.
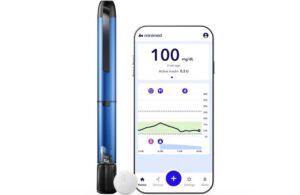
Medtronic wins FDA clearance for MiniMed Go app for InPen smart insulin pen
Medtronic (NYSE: MDT)+
announced today that the FDA granted 510(k) clearance for its MiniMed Go app for multiple daily injections (MDI).
