MedTech News
.................... by Andrew Celentano
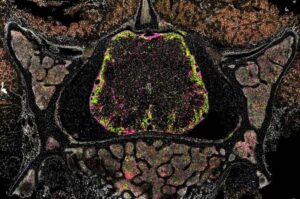
New insight into the immune signals driving inflammation in multiple sclerosis
Multiple sclerosis (MS) is a chronic neurological disease characterized by nerve damage and consequent impairments in vision, movement, balance and mental function. In MS, the immune system mistakenly starts attacking myelin, the protective sheath that surrounds axons (i.e., nerve fibers) in the brain, spinal cord and optic nerves.

‘Unique’ AI-powered headset can predict epilepsy seizures
A “unique” AI-powered headset that can predict epileptic seizures minutes before they occur has been developed by scientists in Scotland.

Genetic study uncovers unknown causes of blindness
Researchers from Radboud University Medical Center and University of Basel have discovered new genetic causes of inherited blindness.
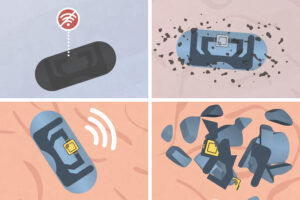
Pills that communicate from the stomach could improve medication adherence
MIT engineers designed capsules with biodegradable radio frequency antennas that can reveal when the pill has been swallowed.

Scientists map the human genome in 4D
Study is a landmark effort to understand how DNA’s physical structure influences human biology
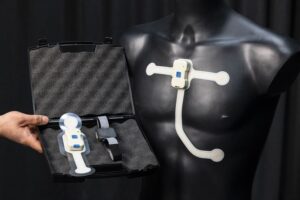
Wearable sensors assist in developing safer and better home dialysis – HUS and VTT start testing with patients
ESPOO, Finland, Jan. 8, 2026 /PRNewswire/ — HUS Helsinki University Hospital and VTT are starting a field test of wearable sensors to develop home dialysis. A total of 36 volunteer patients will use the sensors that enable monitoring of treatment effectivity. Making home dialysis safer and better would improve life quality and save society millions of euros annually.

Saluda Medical Receives Regulatory Approval for EVA™ Sensing Technology in Europe with recognition in Australia
MINNEAPOLIS, Jan. 8, 2026 /PRNewswire/ — Saluda Medical, Inc. (ASX:SLD, “Saluda” or the “Company”), a commercial-stage medical device company focused on developing treatments for chronic neurological conditions using its novel closed-loop neuromodulation platform, announced that, as expected, its next-generation EVA™ Sensing Technology has now received CE certification for commercialization in Europe with recognition of this approval in Australia. This follows FDA approval of EVA in December 2024.
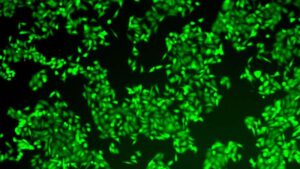
Short-circuiting pancreatic cancer: A potential RNA therapy
Cold Spring Harbor Laboratory (CSHL) Professor Adrian Krainer’s lab discovered how the protein SRSF1 along with AURKA and MYC, jumpstart PDAC tumor development.
