MedTech News
.................... by Andrew Celentano

Online tool detects drug exposure directly from patient samples
As a result, significant drug exposures can be missed. Knowing what drugs are present is important because they can have unexpected effects on biology and health.
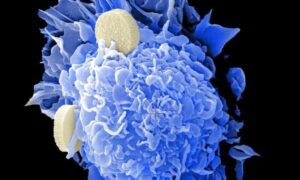
New gene-mapping method unlocks hidden drivers of cancer
University of South Australia scientists have developed a powerful new way to uncover the genetic interactions that fuel cancer progression, paving the way for earlier and more precise treatments.

Allmed Solutions Achieves Landmark ‘Needle-Stick’ Heart Valve Replacement
OR AKIVA, Israel, Dec. 9, 2025 /PRNewswire/ — Allmed Solutions (TASE: ALMD), an Israeli biomedical company, has announced a dramatic success in a first-in-human trial, achieving the full replacement of a major heart valve in critically ill patients via a minimally invasive catheterization procedure, entirely eliminating the need for high-risk open-chest surgery.

Medit Launches AuraVue: A Unified 3D Intraoral Scan and X-Ray Visualization Powered by Overjet AI
NEWPORT BEACH, Calif., Dec. 9, 2025 /PRNewswire/ — Medit, a global leader in 3D intraoral scanning and digital dentistry solutions, today announced the launch of AuraVue, a next-generation visualization and consultation platform that integrates Overjet’s AI technology to deliver real-time diagnostic insights and clearer patient communication.

MedINT Launches First Human-in-the-Loop Medical AI Platform for Complex Clinical Decision-Making
TEL AVIV, Israel, Dec. 9, 2025 /PRNewswire/ — MedINT today announced the commercial launch of the first human-in-the-loop medical AI platform designed to return full control of evidence review and treatment decisions to clinicians managing complex patients. The system maintains clinician control at every step by delivering patient-specific evidence summaries that support clinical judgment without replacing it.
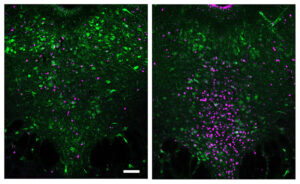
Too sick to socialize: How the brain and immune system promote staying in bed
MIT researchers discover how an immune system molecule triggers neurons to shut down social behavior in mice modeling infection.
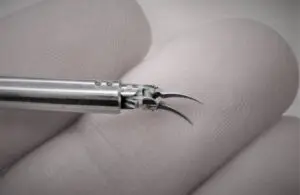
Medical Microinstruments wins FDA nod for surgical robot instruments
Medical Microinstruments (MMI) announced today that the FDA granted 510(k) clearance for its NanoWrist surgical robot instruments.

Aquapass’s fluid overload therapy system gains marketing approval in Israel
The approval is said to cover a range of clinical indications and supports use in both acute and chronic patients.
