MedTech News
.................... by Andrew Celentano
Genetic testing trifecta predicts risk of sudden cardiac death and arrhythmia
New approach could be applied to other complex, genetically influenced diseases like cancer, Parkinson’s
FDA and EU clear Zap Surgical’s radiosurgery planning system
Zap Surgical’s ZAP Axon system aims to make radiosurgery planning more straightforward and efficient.

Go-Pen wins CE mark for user-fillable insulin pen
Go-Pen announced today on social media that it received CE mark approval for its user-fillable insulin pen.
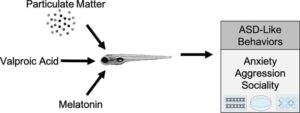
Researchers develop new tool to study autism risk factors
University of Mississippi researchers have developed a new tool to help scientists study how environmental and genetic factors interact to influence autism spectrum disorder.
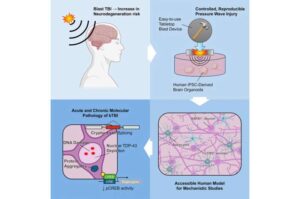
Tabletop blast device brings traumatic brain injury research to the lab bench
Four University of Rhode Island researchers have developed and tested a cost-effective, easy-to-use tabletop device that can generate pressure waves, mimicking the impact of blasts that can cause neurodegeneration. Their study was recently published in the journal Cell Reports Methods. The results will help URI’s Claudia Fallini and Riccardo Sirtori better study the development and progression of neurodegenerative diseases in their lab.
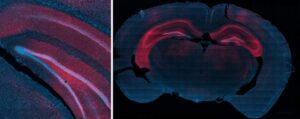
Nonsurgical treatment shows promise for targeted seizure control
Rice University bioengineers have demonstrated a nonsurgical way to quiet a seizure-relevant brain circuit in an animal model. The team used low-intensity focused ultrasound to briefly open the blood-brain barrier (BBB) in the hippocampus, delivered an engineered gene therapy only to that region and later flipped an on-demand “dimmer switch” with an oral drug.
Sentante announces first-of-its-kind remote robotic stroke procedure
Sentante today announced the completion of a first-of-its-kind remote stroke procedure in Scotland using its robotic platform.
Stereotaxis gets FDA nod for next-gen GenesisX surgical robot
Stereotaxis (NYSE:STXS) announced today that it received FDA 510(k) clearance for its next-generation GenesisX surgical robot.
