MedTech News

Photocurrent-responsive coating cuts bone-to-implant integration time in half
A research team has developed an innovative photocurrent-responsive implant surface to accelerate bone-to-implant integration after orthopedic surgery.

Endoquest robotic system gets FDA OK to launch clinical trial
A pivotal study of the robotic platform in colorectal surgery will begin at five hospitals in early 2025.

Wearable patch can monitor breathing to help save lives
Scientists have developed a wearable patch that can accurately monitor and detect changes in people’s breathing—even when not in direct contact with the skin.

Tiny sensors offer new hope for faster bone injury recovery
Tiny implantable sensors are helping University of Oregon researchers optimize the process of recovery from severe bone injuries.
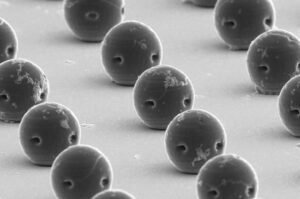
Tiny robots target tumors with precision drug delivery
In the future, delivering therapeutic drugs exactly where they are needed within the body could be the task of miniature robots. Not little metal humanoids or even bio-mimicking robots; think instead of tiny bubble-like spheres.
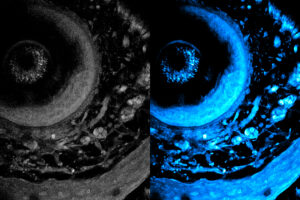
Noninvasive imaging method can penetrate deeper into living tissue
Using high-powered lasers, this new method could help biologists study the body’s immune responses and develop new medicines.

Karl Storz launches AI tool to increase OR efficiency
Karl Storz announced the U.S. launch of Pathway.AI, a new operating room tool offered in partnership with Artisight.

FemPulse wins FDA IDE for for overactive bladder neuromod
FemPulse announced today that it received FDA investigational device exemption (IDE) approval for its overactive bladder (OAB) therapy.
