MedTech News

PENTAX Medical obtained US FDA 510(k) clearance for new models of the PENTAX Medical i20c Video Endoscope Series
MONTVALE, N.J., Dec. 10, 2024 /PRNewswire/ — PENTAX Medical, a division of HOYA Group, has obtained US FDA 510(k) clearance for new models of the PENTAX Medical i20c Video Endoscope Series—PENTAX Medical Video Colonoscope EC34-i20cL, PENTAX Medical Video Upper GI Scope EG27‑i20c and Right/Left Wheel Extender OE-B17.

Using light to monitor blood pressure and track cancer treatment progress
This doesn’t look like a typical blood pressure monitor – but that is exactly what it is.
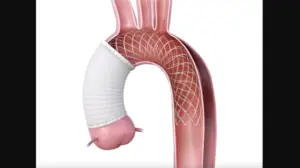
FDA grants humanitarian device exemption to Artivion’s aortic arch remodeling device
Artivion (NYSE:AORT) this week announced it received FDA humanitarian device exemption (HDE) for its AMDS hybrid prosthesis.

EndoQuest wins FDA IDE to study surgical robot in colorectal surgery
EndoQuest Robotics announced today that it received FDA investigational device exemption (IDE) for a study of its surgical robot.

Concord Healthcare Announces Approval Granted to the Application for the Proton Therapy Equipment Registration Certificate
BEIJING, Dec. 9, 2024 /PRNewswire/ — Concord Medical Services Holdings Limited (“Concord Medical” or the “Company”) (NYSE: CCM), a healthcare provider specialized in cancer treatment, research, education and prevention in China, today announced that the National Medical Products Administration of the People’s Republic of China has granted approval to the application for the Registration Certificate for Medical Device for the proton therapy equipment (the “Registration Certificate”) made by the Company’s equipment supplier on December 6, 2024.

Stereotaxis wins approval in China for magnetic ablation catheter
Stereotaxis (NYSE:STXS) announced today that its Magbot magnetic navigation ablation catheter received regulatory approval in China.
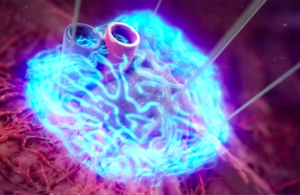
AngioDynamics wins FDA clearance for NanoKnife system in prostate tissue ablation
AngioDynamics today announced it received FDA 510(k) clearance for its NanoKnife system for prostate tissue ablation in patients with intermediate-risk prostate cancer.
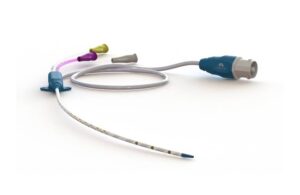
Lungpacer Medical wins FDA premarket approval for AeroPace neurostim system
Lungpacer Medical announced that it received FDA premarket approval for its flagship AeroPace neurostimulation system.
