New smartphone test to ‘revolutionise’ type 2 diabetes prevention
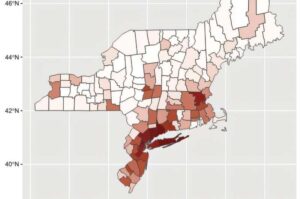
PocDoc, a leading UK digital health diagnostics company, has launched what it says is the world’s first smartphone-based test for type 2 diabetes risk including the gold-standard blood biomarker, HbA1c, a landmark step in diabetes prevention according to the company.