FDA Launches New PreCheck Program to Lower Regulatory Barriers to Pharma Manufacturing
Under PreCheck, the FDA will communicate more frequently with pharmaceutical companies, helping them as they establish or expand manufacturing sites in the U.S.

Under PreCheck, the FDA will communicate more frequently with pharmaceutical companies, helping them as they establish or expand manufacturing sites in the U.S.
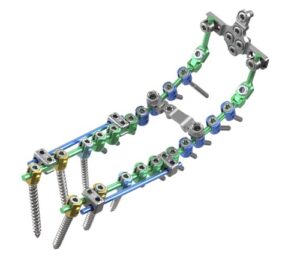
PLANO, Texas, Aug. 7, 2025 /PRNewswire/ — ulrich medical USA, a leading medical device company focused on developing and commercializing spinal implant technologies, is proud to announce the full market launch of the Cortium® Universal OCT Spinal Fixation System, following a successful alpha release.

Modular Medical (Nasdaq:MODD) announced today that it validated its insulin pump cartridge line for human-use production in the U.S.

TWINSBURG, Ohio, July 15, 2025 /PRNewswire/ — Edgepark has recently expanded its portfolio to include the latest innovations from diabetes manufacturers, including the first-ever FDA-cleared yearlong continuous glucose monitor (CGM) system, and a glucose biosensor that can be purchased without a prescription.
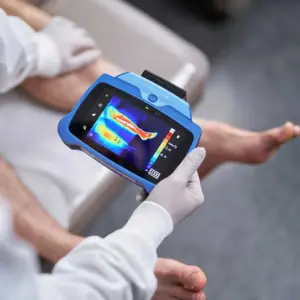
New imaging innovation enhances wound assessment by combining fluorescence and thermal data for improved diagnostic precision and patient care

Sequel Med Tech announced at the American Diabetes Association’s 85th Scientific Sessions in Chicago that it plans to launch its twiist system in July.
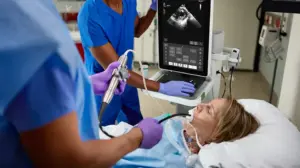
Next-gen portable ultrasound system combines speed, image clarity, and AI-powered tools to elevate diagnostic confidence at the bedside.
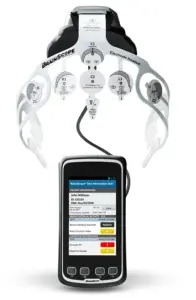
New leadership and AI-driven technology aim to revolutionize assessment and care for brain-related conditions
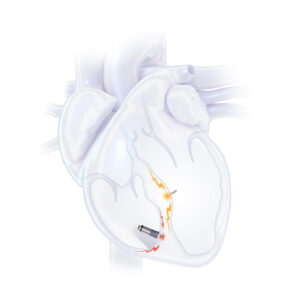
SUNNYVALE, Calif., June 10, 2025 /PRNewswire/ — In a major milestone for heart failure treatment, the first commercial patients in the U.S. have been successfully implanted with the FDA-approved WiSE® System—marking the beginning of a new chapter in leadless left ventricular endocardial pacing (LVEP) for the treatment of heart failure.
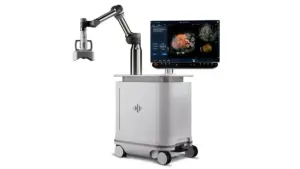
Breakthrough Non-Invasive Therapy Installed with Support from Li Ka Shing Foundation to Advance Research and Patient Care