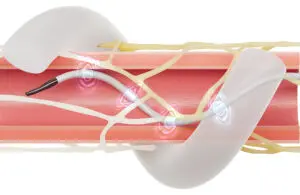
Medtronic achieves renal denervation reimbursement milestone with CMS
Medtronic (NYSE: MDT)+
announced that CMS is opening a national coverage analysis on renal denervation (RDN) procedures for patients with hypertension.

Medtronic (NYSE: MDT)+
announced that CMS is opening a national coverage analysis on renal denervation (RDN) procedures for patients with hypertension.
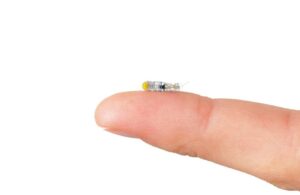
Robeauté announced today that it raised $28 million to support the development of its neurosurgical microrobot technology.

ENGLEWOOD CLIFFS, N.J., Jan. 13, 2025 /PRNewswire/ — BRAIN.Q, is excited to announce the CE Mark for its BQ 2.0 system, designed to reduce disability following ischemic stroke, the number one cause of disability worldwide.

Insulin delivery technology developer CeQur today announced a significant funding round to support its commercialization efforts.

Fire1 announced today that it completed a $120 million financing round to accelerate the advancement of its heart failure management system.

Medtronic (NYSE: MDT)+
today announced CE mark approval for its Harmony transcatheter pulmonary valve (TPV) system
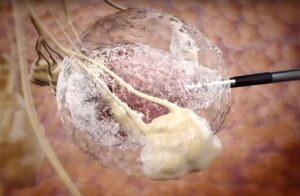
IceCure Medical (Nasdaq:ICCM) announced today that it received an intention to grant notice from the European Patent Office.
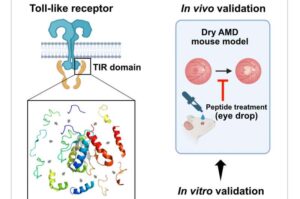
Age-related macular degeneration (AMD) is the leading cause of vision loss in individuals over 65, characterized by abnormal changes in the macular, resulting in reduced vision and distorted objects. Dry AMD accounts for 90% of all AMD cases, with relatively mild vision impairment; however, approximately 30% progress to the severe vision loss associated with wet AMD within 10 years.

Vivani Medical (Nasdaq:VANI) announced today that it initiated screening and enrollment for a first-in-human trial of its GLP-1 implant.

MannKind today announced six-month results from its Phase 3 INHALE-1 study of Afrezza insulin inhalation powder in children.