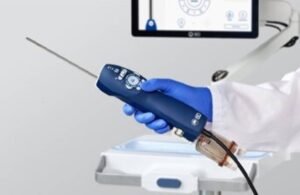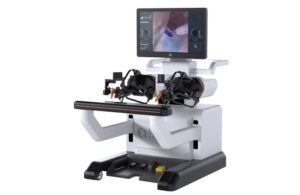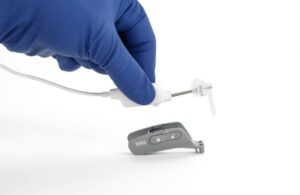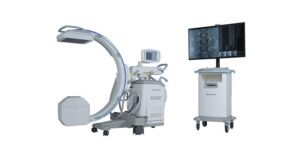
Proprio’s Fourth FDA Clearance Highlights Growing Shift to 3D Measurement in Spine Care
SEATTLE, Jan. 15, 2026 /PRNewswire/ — Proprio, the surgical technology company pioneering real-time, AI-powered intraoperative guidance and data-driven surgical workflows, today announced the U.S. Food and Drug Administration (FDA) has granted clearance for the “Picasso” feature. This marks the company’s fourth FDA-cleared capability within its Paradigm platform.