
Ceribell gets FDA breakthrough nod for stroke detection, monitoring solution
Ceribell (Nasdaq:CBLL) announced today that the FDA granted breakthrough device designation for its large vessel occlusion (LVO) stroke detection monitor.

Ceribell (Nasdaq:CBLL) announced today that the FDA granted breakthrough device designation for its large vessel occlusion (LVO) stroke detection monitor.
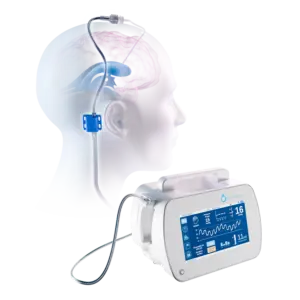
SEATTLE, Jan. 2, 2026 /PRNewswire/ — BrainSpace, a medical technology company building automated solutions for neuro injury, illness and degeneration, today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for Intellidrop.

HAI Solutions, a leading innovator in ultraviolet (UVC) microbial reduction medical devices, announced that the U.S. Food and Drug Administration (FDA) has granted De Novo classification for its QIKCAP System. This marks the first and only FDA-Granted Ultraviolet light-based microbial reduction device for luer-activated valves, establishing a new Class II medical device category.
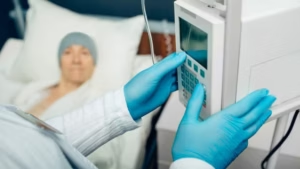
KORU Medical has submitted a 510(k) premarket notification to the US Food and Drug Administration (FDA) seeking approval for its FreedomEDGE infusion system.

MannKind (Nasdaq:MNKD) announced today that the FDA approved its Furoscix (furosemide) on-body infusor for pediatric patients.

Edwards Lifesciences (NYSE: EW)+
has won FDA approval for its Sapien M3 mitral valve replacement system for treating mitral regurgitation (MR).

Shape Memory Medical’s IMPEDE embolisation plug range has received CE mark as a Class III device under the European Union’s Medical Device Regulation (EU MDR).

Diabeloop announced today that its DBLG2 algorithm for automated insulin delivery has received FDA 510(k) clearanc

Stifel analysts said the label for cardiac myosin inhibitor Myqorzo is in line with their expectations and is differentiated compared with BMS’ Camzyos.

Abbott (NYSE: ABT)+
announced today that the FDA approved its Volt pulsed field ablation (PFA) system to treat patients with AFib.