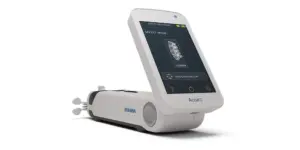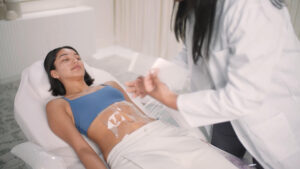
Cutera® Announces New FDA Clearances for truFlex® to Support Rehabilitation, Recovery and Muscle Wellness
The next evolution of truFlex fortifies its position as a versatile solution for rehabilitation with new applications in functional strength for the abdomen, arms, calves, glutes and thighs.