
BlueWind wins FDA nod for enhanced urinary incontinence wearable
BlueWind Medical announced today that the FDA granted 510(k) clearance for its enhanced Revi implantable tibial neuromodulation (iTNM) system.

BlueWind Medical announced today that the FDA granted 510(k) clearance for its enhanced Revi implantable tibial neuromodulation (iTNM) system.
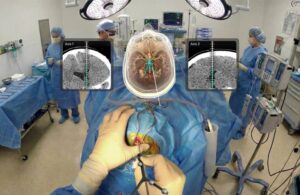
Medivis announced today that it received FDA 510(k) clearance for its augmented reality (AR)-powered Cranial Navigation platform.

The Anavi system treats polycystic ovary syndrome by delivering targeted radiofrequency energy to ablate ovarian tissue to re-initiate ovulatory cycles.

The patch is tailored to deliver all types of vaccines to the skin using a simple applicator.
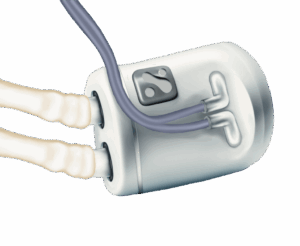
The FDA has granted Breakthrough Device Designation to Nephrodite’s Holly implantable continuous dialysis system.

TAMPERE, Finland, Dec. 14, 2025 /PRNewswire/ — Bioretec Oy (“Bioretec” or the “Company”), a pioneer in biodegradable orthopedic implants, has been granted Breakthrough Device Designation by the U.S. Food and Drug Administration (FDA) for its magnesium alloy technology-based, biodegradable RemeOs™ DrillPin.

HONG KONG, Dec. 12, 2025 /PRNewswire/ — Peijia Medical (9996.HK), a leading Chinese domestic company in the high-growth transcatheter valve therapeutics and neurovascular interventions markets, announced that its TaurusTrio Transcatheter Aortic Valve (TAV) system received approval from the National Medical Products Administration (NMPA) of China on December 11, 2025.
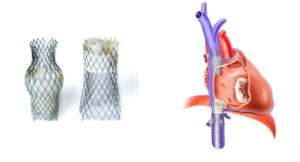
P&F USA, Inc., the U.S. subsidiary of heart valve manufacturer P&F Products and Features GmbH, today announced that the U.S. Food and Drug Administration (FDA) has approved initiation of the TRICAV II Pivotal Trial.

The Bendit17 steerable microcatheter aims to advance navigational control through complex anatomies in vascular procedures.
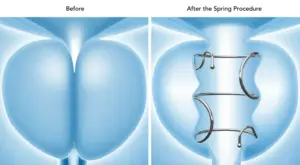
The Spring is the only FDA-approved FIT in a range of lengths and diameters, allowing for patient personalisation