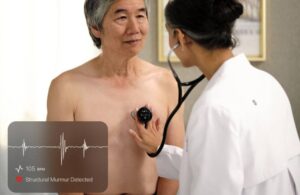
Eko reports reimbursement win for AI-powered cardiac disease detection
Eko Health announced today that the Centers for Medicare & Medicaid Services (CMS) has finalized national payment for its Sensora platform.

Eko Health announced today that the Centers for Medicare & Medicaid Services (CMS) has finalized national payment for its Sensora platform.

MannKind (Nasdaq:MNKD) announced that the FDA accepted a supplemental New Drug Application (sNDA) for its Furoscix ReadyFlow autoinjector.

The decision is supported by evidence from a clinical study and data from supporting analytical validation trials.

Medical Microinstruments (MMI) announced today that it won reimbursement for its surgical lymphovenous bypass (LVB) surgery system.
The a2z-Unified-Triage device is designed to simultaneously flag and prioritise seven urgent findings on abdomen-pelvis CT scans.

GE HealthCare’s Recon DL is claimed to be the first mammography technology to use deep learning alongside iterative reconstruction to improve image quality.

BD Surgiphor™ is a sterile, ready-to-use irrigation solution designed to help loosen and remove debris from surgical wounds during procedures
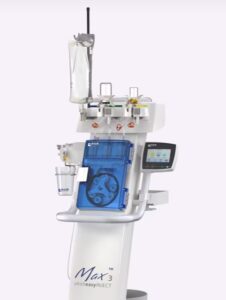
PRINCETON, N.J., Nov. 25, 2025 /PRNewswire/ — Bracco Diagnostics Inc., the U.S. subsidiary of Bracco Imaging S.p.A., a global leader in diagnostic imaging, announced today that the U.S. Food and Drug Administration (FDA) has expanded the indication for the Bracco-branded Max 3™, a Rapid Exchange and Syringeless Injector for use in magnetic resonance imaging (MRI) procedures. In addition to single-dose and multi-dose vials, the Max 3™ system is now indicated for use with the newly approved VUEWAY® (gadopiclenol) injection Imaging Bulk Package (IBP) in 30 mL and 50 mL formats.
Transmural Systems’ Telltale guide wire aims to prevent coronary obstruction by modifying the ‘leaflet’ tissue in high-risk patients undergoing TAVR.

The Centers for Medicare and Medicaid Services (CMS) has provided a major reimbursement update for cardiac ablation technologies.