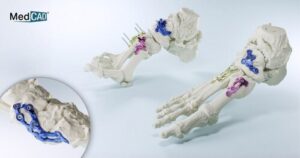
MedCAD’s AccuStride™ Fixation Plates Receive 510(k) FDA Clearance to Complete Foot and Ankle System Offering
DALLAS, Nov. 6, 2025 /PRNewswire/ — Dallas-based MedCAD has been awarded 510(k) FDA clearance for its AccuStride fixation plates, part of a patient-specific solution available to surgeons as a complete foot and ankle (F&A) system. The company received 510(k) FDA clearance for its foot and ankle guides and planning system in March 2025. The AccuStride implant and surgical guide system coupled with the company’s proprietary planning software will enable foot and ankle surgeons to correct multiple related pathologies in a single procedure.