FDA grants 510(k) clearance to Sirona Medical’s imaging suite
The clearance from the US regulator broadens the company’s diagnostic imaging offerings.

The clearance from the US regulator broadens the company’s diagnostic imaging offerings.
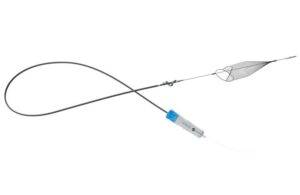
InterVene announced today that it received FDA 510(k) clearance for its Recana thrombectomy catheter system.
Studies showed that the assay demonstrated a 94.90% sensitivity in polymerase chain reaction-confirmed positive samples.

GROVE CITY, Ohio, Oct. 30, 2025 /PRNewswire/ — Tosoh Bioscience, Inc., a market leader in clinical diagnostics has received U.S. FDA 510(k) clearance for its next-generation Tosoh Automated Glycohemoglobin Analyzer HLC®-723 GR01 (GR01) for HbA1c testing.

National Medicare coverage paves the way for a larger patient population to access the blood pressure treatment, which gained FDA approval in 2023.

REDMOND, Wash., Oct. 28, 2025 /PRNewswire/ — Perimetrics, Inc., a dental technology company pioneering quantitative diagnostics, announced today that the U.S. Food and Drug Administration (FDA) has granted clearance for the InnerView® System — the first FDA-cleared technology designed to measure both internal and external mobility in teeth and implants.

Zimmer Biomet (NYSE: ZBH)+
announced today that it received FDA breakthrough device designation for its iodine-treated total hip replacement system.

BEAVERTON, Ore., Oct. 24, 2025 /PRNewswire/ — YorLabs, Inc., a medical technology company pioneering next-generation intracardiac imaging solutions for electrophysiology (EP) and interventional cardiology (IC) procedures, today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the company’s YorLabs Intracardiac Imaging System – a first-of-its-kind, zero-capex ultrasound platform designed to simplify workflow, reduce cost, and enhance procedural efficiency inside the cath lab.

The expanded FDA clearance makes NeurAxis’ IB-Stim the first FDA approval for a treatment that specifically addresses functional dyspepsia.

Edge Medical announced on LinkedIn today that it received CE mark for MSP2000, its robotic-assisted “super system.”