
Virtuoso Surgical wins FDA breakthrough device designation for surgical robot
Virtuoso Surgical announced today that it received FDA breakthrough device designation for bladder lesion removal with its surgical robot.

Virtuoso Surgical announced today that it received FDA breakthrough device designation for bladder lesion removal with its surgical robot.
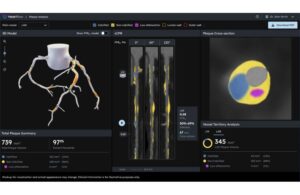
Heartflow (Nasdaq:HTFL) announced that it received FDA 510(k) clearance for its next-generation Heartflow Plaque Analysis algorithm.
Techsomed this week announced it received FDA 510(k) clearance to expand indications for its BioTraceIO360 software platform to include percutaneous ablation of soft tissue in the kidney.

Medtronic (NYSE: MDT)+
announced today that it received FDA approval for its Altaviva neuromodulation device.
CAESAREA, Israel, Sept. 18, 2025 /PRNewswire/ — IceCure Medical Ltd. (NASDAQ: ICCM) (“IceCure”, “IceCure Medical” or the “Company”), developer of minimally-invasive cryoablation technology that destroys tumors by freezing as an option to surgical tumor removal, today announced it has received a Notice of Allowance from the U.S. Patent and Trademark Office for its patent titled “Cryoprobe”.
The company is working to obtain CE marking of the system in the European Union.
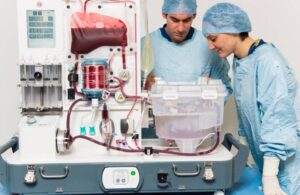
OrganOx announced today that it received FDA approval for the use of its normothermic machine perfusion (NMP) device during air transport.
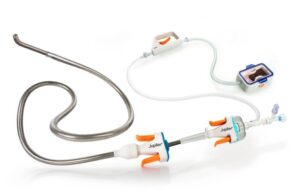
Jupiter Endovascular announced today that its Vertex catheter received FDA 510(k) clearance for the insertion of endovascular devices.
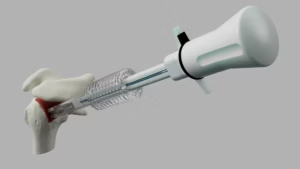
Durham, NC — September 16, 2025 — SutureTech, a surgeon-founded medical device company focused on advancing soft tissue and tendon repair, today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for RapidFix™, its flagship device.
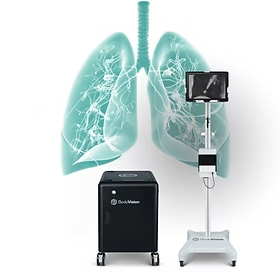
WALTHAM, Mass., Sept. 16, 2025 /PRNewswire/ — Body Vision Medical, a global leader in AI-powered intraoperative imaging, is proud to announce that its LungVision AI-powered advanced imaging system has received regulatory approval from the Central Drugs Standard Control Organization (CDSCO) in India. This approval marks a significant milestone in the company’s mission to improve early and accurate lung cancer diagnosis worldwide.