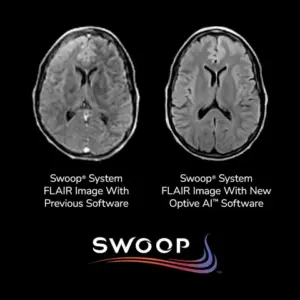
IceCure Receives Notice of Patent Allowance in U.S. for a Novel Cryogen Flow Control to Optimize Patient Outcomes
IceCure has 20+ patents in the U.S. and the Company anticipates further market traction upon FDA’s marketing authorization decision in early-stage breast cancer