
Distalmotion wins FDA nod for gallbladder removal with surgical robot
Distalmotion announced today that it received FDA 510(k) clearance for the use of its Dexter surgical robot in adult cholecystectomy (gallbladder removal).

Distalmotion announced today that it received FDA 510(k) clearance for the use of its Dexter surgical robot in adult cholecystectomy (gallbladder removal).
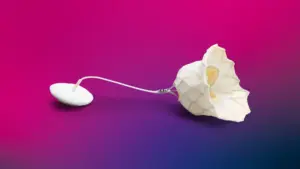
Abbott said the Tendyne system addresses a significant unmet need, providing an option for patients ineligible for open heart surgery and whose valves cannot be successfully repaired with MitraClip.
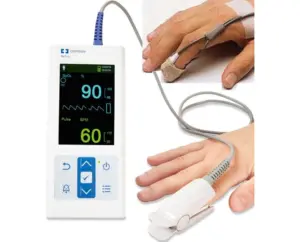
Medtronic (NYSE: MDT)+
announced today that the FDA accepted its new Nellcor technology into its STeP program.

The company said in a news release that the latest clearance marks a significant step forward in gastrointestinal (GI) motility monitoring. It offers clinicians an advanced, non-invasive tool for assessing whole-gut transit times with unmatched accuracy and patient comfort.

CENTER VALLEY, Pa., May 27, 2025 /PRNewswire/ — Olympus, a global medical technology company committed to making people’s lives healthier, safer and more fulfilling, announced today FDA 510(k) clearance of its EZ1500 series endoscopes featuring Extended Depth of Field (EDOF™) technology.

Connected monitoring device brings real-time, remote care support for pregnant patients and individuals at cardiovascular risk.

Breakthrough endovascular device advances minimally invasive arteriovenous graft creation for patients requiring hemodialysis.

Medtronic (NYSE: MDT)+
announced today that it received CE mark for several expanded indications for its Prevail balloon catheter

AI-driven system targets real-time, lab-quality disease detection at the point of care
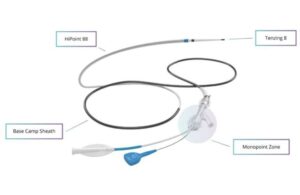
Route 92 Medical announced today that it received FDA 510(k) clearance for its HiPoint reperfusion system.