
Lungpacer wins FDA IDE for AeroNova system
Lungpacer Medical announced today that it received FDA investigational device exemption (IDE) to begin a trial for its AeroNova system.

Lungpacer Medical announced today that it received FDA investigational device exemption (IDE) to begin a trial for its AeroNova system.

BREA, Calif., March 10, 2025 /PRNewswire/ — Beckman Coulter Diagnostics, a clinical diagnostics leader, today announced that the new DxC 500i Clinical Analyzer, an integrated clinical chemistry and immunoassay analyzer, received 510(k) clearance from the U.S. Food and Drug Administration. The DxC 500i combines advanced technology with an intuitive user interface, ensuring that laboratories of all sizes can meet the growing demands of modern healthcare. With throughput of up to 800 clinical chemistry tests per hour and 100 immunoassay tests per hour, this analyzer delivers precise and reliable results critical for timely clinical decision-making.

Zimmer Biomet (NYSE: ZBH) announced today that it received FDA 510(k) clearance for its Persona Revision SoluTion Femur.

Phantom Neuro today announced it received FDA breakthrough device designation and Targeted Acceleration Pathway (TAP) designation for its minimally invasive neural interface Phantom X.

MINNEAPOLIS and MISGAV, Israel, Feb. 27, 2025 /PRNewswire/ — Arcuro Medical Ltd., (“Arcuro”) today announced that it received FDA 510(k) clearance for its new SuperBall-RC™ system for use in rotator cuff repair procedures.
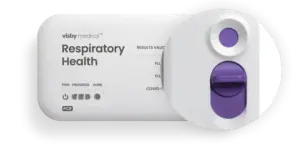
SAN JOSE, Calif., Feb. 26, 2025 /PRNewswire/ — Visby Medical™ announced today that it has received 510(k) clearance and was granted a CLIA waiver from the U.S. Food and Drug Administration (FDA) for its point-of-care respiratory health test. The Visby Medical Respiratory Health Test is a rapid polymerase chain reaction (PCR) test that detects and differentiates between upper respiratory infections caused by influenza (Flu) A & B, and SARS-CoV-2 (COVID-19). This multiplexed molecular device is the first handheld test to receive this designation after being granted Emergency Use Authorization (EUA) in December 2022.

TULSA, Okla., Feb. 26, 2025 /PRNewswire/ — Sway Medical, Inc., the company that created the Mobile Concussion Management category, is proud to announce that it has received FDA 510(k) clearance as a Computerized Cognitive Assessment Aid for Concussion under Section 882.1471. This clearance expands on Sway Medical’s previous FDA clearance for balance testing in head injuries, officially recognizing Sway as the first fully integrated tool that combines both cognitive and balance testing into one product for concussion management.

PALM COAST, Fla., Feb. 25, 2025 /PRNewswire/ — OrthoNovis, Inc is proud to announce that it has received U.S. Food and Drug Administration (FDA) clearance to market its innovative BPS Wrist Fracture System. www.orthonovis.com
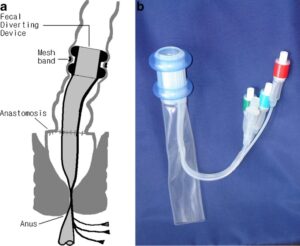
SAN FRANCISCO, Feb. 25, 2025 /PRNewswire/ — Averto Medical, a clinical-stage medical device company pioneering minimally invasive gastrointestinal care, is pleased to announce that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for its ColoSeal™ Intraluminal Colonic Diversion (ICD) System. This designation underscores the potential of ColoSeal™ to significantly improve outcomes for patients undergoing colorectal surgery by eliminating the need for a temporary ostomy.

Tandem Diabetes Care (Nasdaq:TNDM) today announced a major regulatory win that brings its diabetes technology to more patients.