InnoVoyce Achieves ISO 13485 Certification, Announces Nationwide Availability of VYLO™ Blue Light Laser System
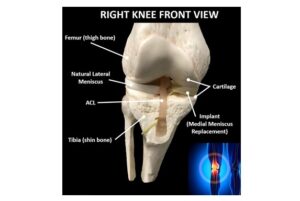
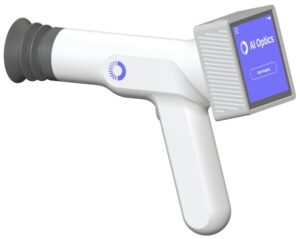
BOSTON, Feb. 11, 2025 /PRNewswire/ — InnoVoyce, a leading medical technology company, proudly announces its ISO 13485:2016 certification, underscoring its dedication to excellence in the design, manufacturing, installation, and servicing of lasers and fiber devices. Alongside this milestone, the company is thrilled to share that its FDA-cleared VYLO™ 455nm Blue Light Laser System is now available nationwide, offering healthcare professionals an advanced tool for treating laryngeal disorders and a wide range of surgical applications.