SeeMedX Files 510(k) with FDA for Innovative Cardiac Monitoring System to Transform Heart Failure Care
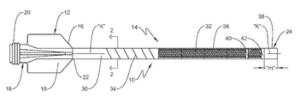
LAS VEGAS, Nov. 4, 2024 /PRNewswire/ — SeeMedX Inc. (“SeeMedX”) today announced the submission of its 510(k) premarket notification to the U.S. Food and Drug Administration (FDA) for its innovative, non-invasive cardiac monitoring device, designed to allow medical professionals to have real-time insights into cardiac performance and fluid status