
Machina Medical Receives FDA Clearance for MFUSE External Bleeding Control Device
MFUSE is designed to support clinicians in managing external bleeding by forming a flexible hydrogel barrier when applied to the wound site

MFUSE is designed to support clinicians in managing external bleeding by forming a flexible hydrogel barrier when applied to the wound site

SurGenTec’s ION-C system is now indicated for the treatment of cervical pseudoarthrosis when implanted bilaterally within the facet joints.
The Xpert GI Panel identifies pathogens directly from stool specimens collected in Cary-Blair transport media.

mOm Incubators announced today that the FDA granted 510(k) clearance for its Essential Incubator system.
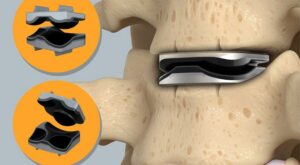
MORGANTOWN, W.Va., Jan. 20, 2026 /PRNewswire/ — Omnia Medical, a medical technology company developing surgical solutions for spine and interventional pain physicians, today announced the commercial launch of its FDA-cleared PsiF DNA™ Sacroiliac Joint Stabilization System.

FDA-cleared PCR test aids in detection of 11 diarrhea-causing bacteria, viruses, and parasites from 1 sample

The FDA-approved comprehensive genomic profiling test will be reimbursed at a rate of $2,989.55 per test, helping to advance adoption in the US healthcare system
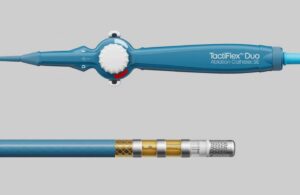
Abbott (NYSE: ABT)+
announced today that it received CE mark for its TactiFlex Duo Ablation Catheter, Sensor-Enabled, to treat AFib.
Amplifi Vascular announced that it received breakthrough device designation for its Amplifi Vein Dilation System.
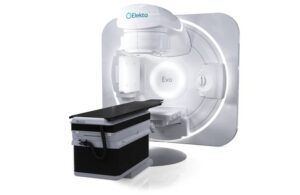
Elekta announced today that its Evo CT linear accelerator (linac) received FDA 510(k) clearance, making it available to U.S. radiation oncology professionals.