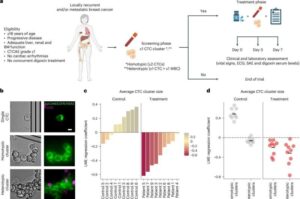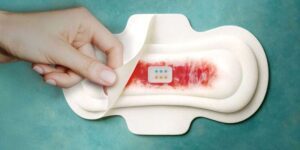
Smart sanitary pads use rapid test strips and AI app to reveal key health biomarkers
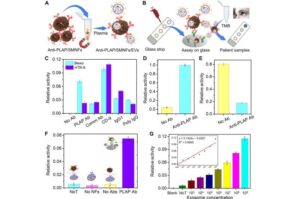
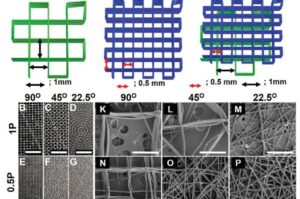
Researchers at ETH Zurich have developed the first technology that is able to recognize biomarkers in menstrual blood—directly in sanitary towels. MenstruAI promises a simple, non-invasive method for recording health data in everyday life.