
May Health gains CE mark for infertility treatment
The Anavi system treats polycystic ovary syndrome by delivering targeted radiofrequency energy to ablate ovarian tissue to re-initiate ovulatory cycles.

The Anavi system treats polycystic ovary syndrome by delivering targeted radiofrequency energy to ablate ovarian tissue to re-initiate ovulatory cycles.

TAMPERE, Finland, Dec. 14, 2025 /PRNewswire/ — Bioretec Oy (“Bioretec” or the “Company”), a pioneer in biodegradable orthopedic implants, has been granted Breakthrough Device Designation by the U.S. Food and Drug Administration (FDA) for its magnesium alloy technology-based, biodegradable RemeOs™ DrillPin.

A new, soft, all-in-one, wearable system has been designed for continuous wireless monitoring of neonatal health in low-resource settings.

The assay is currently available in nations that recognise the CE Mark.
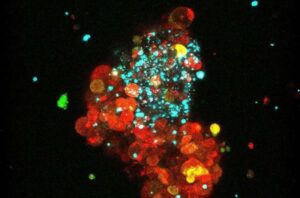
A study led by researchers at the Hospital del Mar Research Institute advances one of the most significant milestones in breast cancer treatment, making immunotherapy effective against the most common tumor type, estrogen receptor-positive or luminal breast cancer.

Triple-negative breast cancer (TNBC) is one of the most aggressive and treatment-resistant forms of breast cancer.
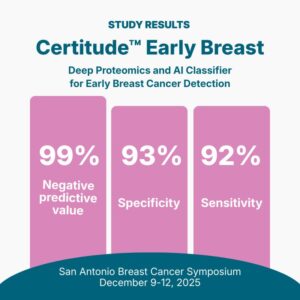
ST. PAUL, Minn., Dec. 3, 2025 /PRNewswire/ — Astrin Biosciences, a cancer intelligence company transforming how cancer is detected and treated through deep proteomics and AI, announced the launch of Certitude™, the first-of-its-kind, non-imaging, blood-based early cancer detection test. Results from the latest study will be presented at the upcoming San Antonio Breast Cancer Symposium (SABCS), taking place December 10–14, 2025.

A new analysis of research into the most common type of breast cancer has zeroed in on an overlooked hormone that may be responsible for the increased risk of breast cancer death in postmenopausal women with obesity.

Scientists at The University of Texas at El Paso have found a promising new target in the fight against high-grade serous carcinoma, an aggressive form of ovarian cancer. Less than 50% of women survive five years after diagnosis, according to the team.
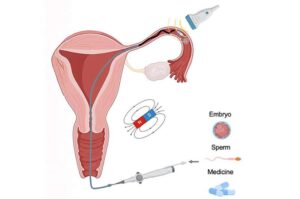
A new international study led by the Nanobiosystems group at CIC nanoGUNE, is developing miniature, non-invasive, precise robotic catheters for use in reproductive medicine and gynecological health.