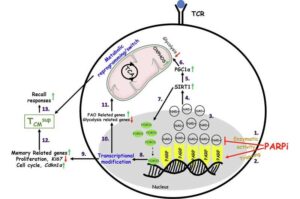
T cells gain superior memory through new reprogramming method, boosting cancer-fighting abilities
Georgetown University’s Lombardi Comprehensive Cancer Center researchers have identified a new way to reprogram T cells, which are infection and tumor-fighting white blood cells, so that they have a superior memory, thereby making them more effective in killing cancer cells.