
Senseonics launches automated insulin dosing system with 1-year CGM
The company has integrated its Eversense 365 implant with Sequel Med Tech’s insulin delivery system to automate the management of Type 1 diabetes.

The company has integrated its Eversense 365 implant with Sequel Med Tech’s insulin delivery system to automate the management of Type 1 diabetes.

Staar Surgical (Nasdaq:STAA) announced that it received FDA approval for an expanded age indication for its Evo lenses.

Philips (NYSE: PHG)+ announced that it launched the InkSpace Imaging Snuggle pediatric body array coil for its 3.0T MRI systems.
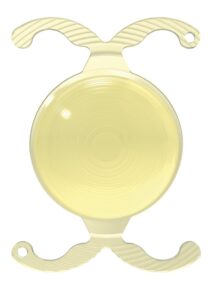
BVI announced the first successful U.S. implantations of the FDA-approved FineVision HP hydrophobic trifocal intraocular lens (IOL).

PLANO, Texas, Feb. 10, 2026 /PRNewswire/ — Vesalio, a global leader in vascular intervention, today announced CE Mark certification and the European commercial launch of two new neurovascular devices: NeVa™ VS, for the treatment of cerebral vasospasm following aneurysmal subarachnoid hemorrhage (aSAH), and the NeVa™ 3.0 mm Thrombectomy System for stroke. In addition, the Company received an additional U.S. Food and Drug Administration (FDA) 510(k) clearance expanding the indications of its neurovascular and peripheral aspiration catheters to include distal access.
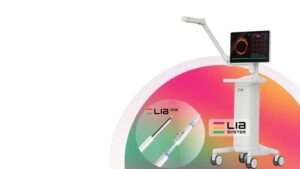
SAN JOSE, Calif., Feb. 5, 2026 /PRNewswire/ — LEADOPTIK, Inc., a medical technology company focused on advancing precision lung cancer biopsy, today announced the successful first-in-human clinical use of its FDA-cleared Last Inch Assessment™ (LIA) System at University of Chicago Medical Center.

Insulet (Nasdaq:PODD) announced today that it launched its Omnipod 5 and Omnipod Discover offerings in the Middle East.

AVENTURA, Fla., Feb. 4, 2026 /PRNewswire/ — Nuvo Intl Group Inc. today announced that pregnant moms can lease the FDA-cleared INVU wearable solution , a cloud-based pregnancy monitoring platform designed for use at home or at work, directly through their physician or healthcare provider.

Dexcom (NSDQ:DXCM) announced today that it plans to launch an advanced AI-enabled enhancement to its Stelo over-the-counter CGM.
Quest expects the new blood test will support response monitoring in clinical trials.