Medtronic gets FDA nod for Infuse bone graft in TLIF procedures
Medtronic (NYSE: MDT)+ announced today that it received FDA premarket approval (PMA) for the use of its Infuse bone graft in TLIF procedures.

Medtronic (NYSE: MDT)+ announced today that it received FDA premarket approval (PMA) for the use of its Infuse bone graft in TLIF procedures.
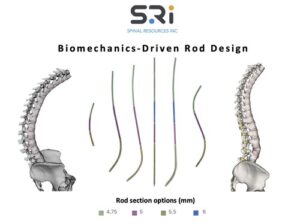
FORT LAUDERDALE, Fla., Feb. 5, 2026 /PRNewswire/ — The Bezier Parametric Curve Rod System from Spinal Resources, Inc. has received 510(k) clearance for compatibility with any cleared pedicle screw set available on the US market, regardless of manufacturer.
Zimmer Biomet (NYSE: ZBH)+
announced today that it received FDA 510(k) clearance for its G7 acetabular system for hip replacement surgeries.

YOUNGSTOWN, Ohio, Jan. 29, 2026 /PRNewswire/ — Nivalon Medical Technologies Inc. has successfully produced the world’s first fully patient-specific, motion-preserving spinal implant built entirely without metal, using AI-driven design and advanced ceramic 3D printing.
The FDA has authorised up to 15 clinical sites to take part in the study.

CORONADO, Calif., Jan. 26, 2026 /PRNewswire/ — Spine Innovation, LLC, a medical device startup that develops novel interbody fusion implants, announced today that is has received FDA 510(k) clearance to market the LOGIC™ Titanium Implant System.
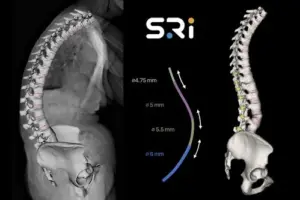
The patent’s focus on a constant-slope transition ensures a gradual and predictable stiffness profile
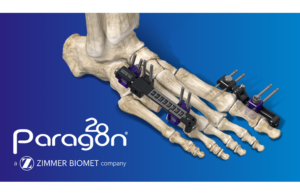
Zimmer Biomet (NYSE: ZBH)+
recently announced the Brachiator mini-rail external fixation system.

SurGenTec’s ION-C system is now indicated for the treatment of cervical pseudoarthrosis when implanted bilaterally within the facet joints.
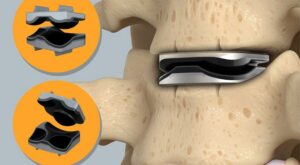
MORGANTOWN, W.Va., Jan. 20, 2026 /PRNewswire/ — Omnia Medical, a medical technology company developing surgical solutions for spine and interventional pain physicians, today announced the commercial launch of its FDA-cleared PsiF DNA™ Sacroiliac Joint Stabilization System.