
Stryker launches Incompass total ankle system
Stryker (NYSE: SYK)+
today announced the launch of its recently FDA-cleared Incompass total ankle system.

Stryker (NYSE: SYK)+
today announced the launch of its recently FDA-cleared Incompass total ankle system.
Varian announced its Embozene microspheres have received CE mark for genicular artery embolisation (GAE) to treat knee osteoarthritis.
ARC‑EX is the first system to receive a CE Mark in Europe specifically for improving hand and arm strength and sensation in adults with chronic, incomplete spinal cord injury.

Researchers have developed a material that can sense tiny changes within the body, such as during an arthritis flareup, and release drugs exactly where and when they are needed.

Lifeward (Nasdaq:LFWD) announced today that it received CE mark approval for its ReWalk 7 personal exoskeleton.

Having lived with an ALS diagnosis since 2018, Kate Nycz can tell you firsthand what it’s like to slowly lose motor function for basic tasks. “My arm can get to maybe 90 degrees, but then it fatigues and falls,” the 39-year-old said. “To eat or do a repetitive motion with my right hand, which was my dominant hand, is difficult. I’ve mainly become left-handed.”

The FDA has granted breakthrough device designation to SpinaFX Medical’s Triojection system, a minimally invasive treatment for contained lumbar disc herniations.
Interventional Systems and HICREN today announced approval for their joint venture’s surgical robot platform in China.
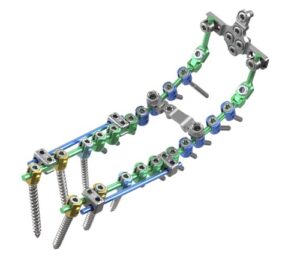
PLANO, Texas, Aug. 7, 2025 /PRNewswire/ — ulrich medical USA, a leading medical device company focused on developing and commercializing spinal implant technologies, is proud to announce the full market launch of the Cortium® Universal OCT Spinal Fixation System, following a successful alpha release.

https://medtechspectrum.com/news/40/24634/mirus-secures-ntap-approval-from-cms-for-breakthrough-europa-cervical-spine-system.html