Carlsmed earns Medicare win for AI-powered spine implants
Carlsmed (Nasdaq:CARL) today announced new technology add-on payment (NTAP) reimbursement for its spine implant technology.

Carlsmed (Nasdaq:CARL) today announced new technology add-on payment (NTAP) reimbursement for its spine implant technology.

NAPLES, Fla., July 16, 2025 /PRNewswire/ — Arthrex, a global leader in minimally invasive surgical technology, announced that it has received U.S. Food and Drug Administration (FDA) clearance to use the Arthrex NanoScope™ operative arthroscopy system for pediatric orthopedics and laparoscopy.
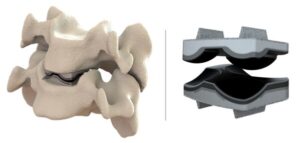
OREM, Utah, July 10, 2025 /PRNewswire/ — Dymicron®, a privately held medical device company pioneering advanced spinal technologies, today announced that the United States (U.S.) Food and Drug Administration (FDA) has granted Investigational Device Exemption (IDE) approval to begin a pivotal clinical study of the Triadyme®–C cervical artificial disc.

Oxiplex is the only FDA authorized intraoperative gel indicated as an adjunct to lumbar spinal surgery to reduce postoperative leg pain and neurological symptoms.

Next-Generation Ankle Replacement Platform Designed to Improve Surgical Precision and Patient Outcomes in Orthopaedic Care
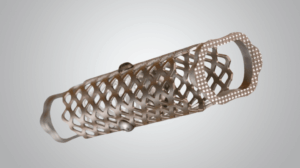
MOUNTAIN VIEW, Calif., June 18, 2025 /PRNewswire/ — Expanding Innovations™ (EI), a rapidly growing spine company specializing in NON-SCREW-based Expandable Technology, today announced U.S. FDA 510(k) clearance for its N-GAGE™ Lumbar Plate System—the company’s first spinal fixation platform as it continues to broaden its procedure-based solutions.
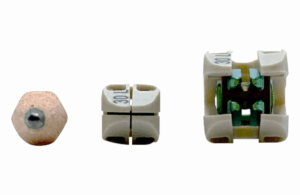
Accelus today announced it received FDA 510(k) clearance for its FlareHawk Interbody Fusion System to be safely scanned under certain MRI conditions
Next-generation platform integrates 3D visualization and smart surgical tools to improve outcomes in shoulder arthroplasty

Trax Surgical today announced it received FDA 510(k) clearance to market its Linkt Compression Staple System.

Think Surgical today announced the first successful use of Waldemar Link’s LinkSymphoKnee using the TMINI Miniature Robotic System.