
FDA expands indications for Wandercraft robotic exoskeleton
Wandercraft announced today that the FDA expanded the indications for its flagship Atalante X rehabilitation device.

Wandercraft announced today that the FDA expanded the indications for its flagship Atalante X rehabilitation device.
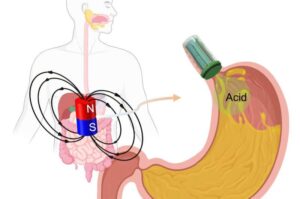
A team of researchers led by Xiaoguang Dong, assistant professor of mechanical and biomedical engineering at Vanderbilt University, have developed a magnetic robotic valve to provide minimally invasive intervention for gastroesophageal reflux disease and possibly other organ system disorders.

Edge Medical announced on LinkedIn today that it received CE mark for MSP2000, its robotic-assisted “super system.”
https://www.medtechdive.com/news/stereotaxis-teams-with-cardiofocus-to-develop-robotic-pfa-system/802799/
Remedy Robotics announced that it debuted the N1 system, a remotely operable endovascular surgical robotic system.
Medical Microinstruments Inc. (MMI) says its robotics system has been used for the first microsurgical intracranial brain surgeries.
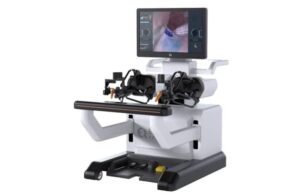
EndoQuest Robotics recently announced the completion of the first procedure performed by a gastroenterologist in its PARADIGM trial.
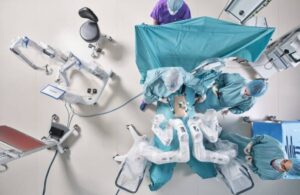
Distalmotion announced today that it received FDA 510(k) clearance for the use of its Dexter robotic surgery system in hysterectomy.

For many stroke survivors, even the smallest number of steps carries enormous weight. Each movement becomes a reminder of lost coordination, muscle weakness, and physical vulnerability.

Virtuoso Surgical announced today that it received FDA breakthrough device designation for bladder lesion removal with its surgical robot.