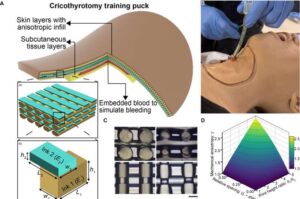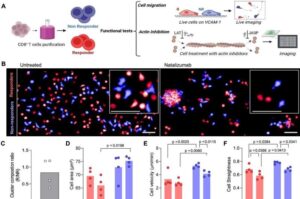
Machine learning and cell imaging combine to predict effectiveness of multiple sclerosis medication
Brazilian researchers, in partnership with French institutions, have developed a tool that can predict how patients will respond to natalizumab, one of the most commonly used drugs for treating multiple sclerosis.